Redacci. Pen sula 360 Press [P360P]
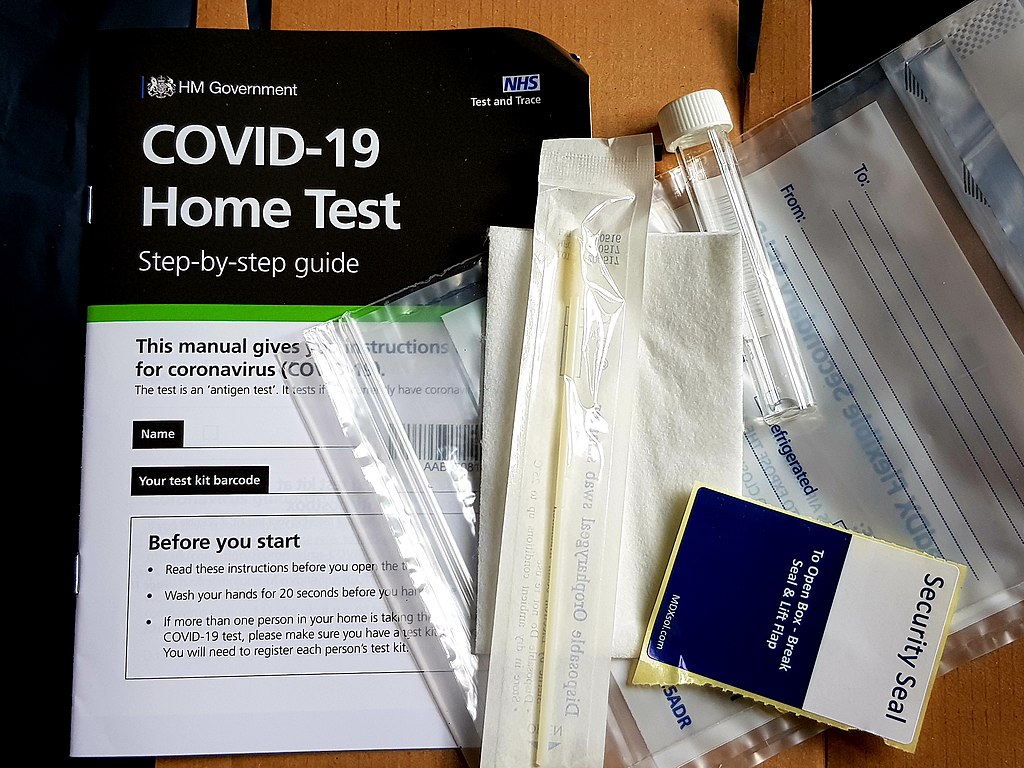
The U.S. Food and Drug Administration (FDA) has issued an emergency use authorization for the first self-test for COVID-19 at home that provides reliable results.
The test kit

The U.S. Food and Drug Administration (FDA) has issued an emergency use authorization for the first self-test for COVID-19 at home that provides reliable results.
The test kit