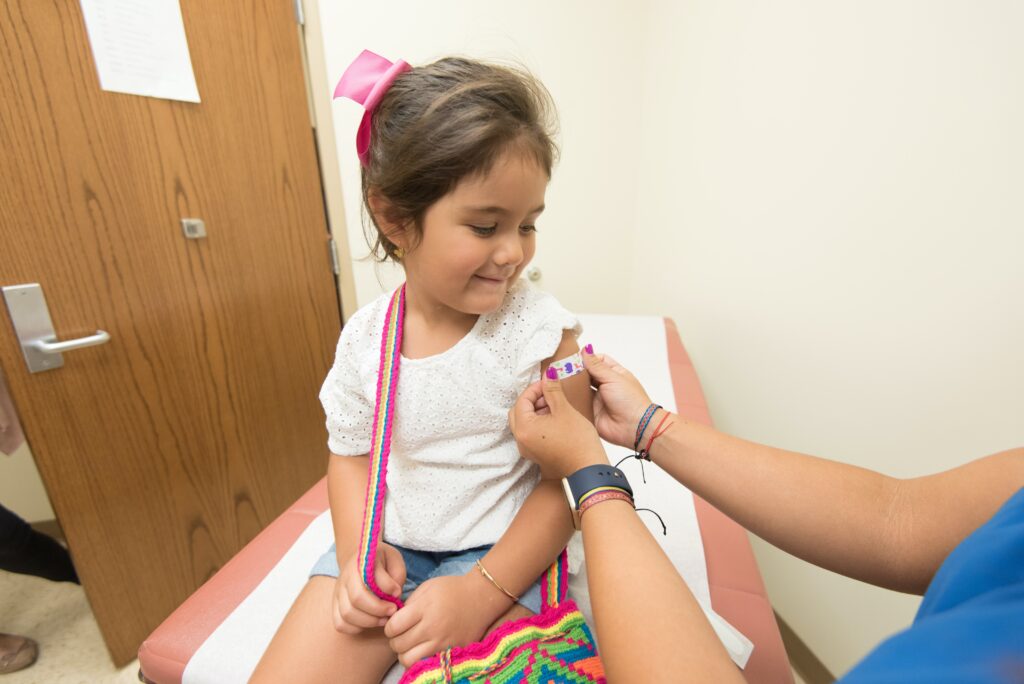
The U.S. Food and Drug Administration The FDA on Thursday, December 8, modified the emergency use authorizations (EUA) for the bivalent boosters of the Moderna and Pfizer-BioNTech COVID-19 vaccines to include use in children up to 6 months to 4 years of age.
