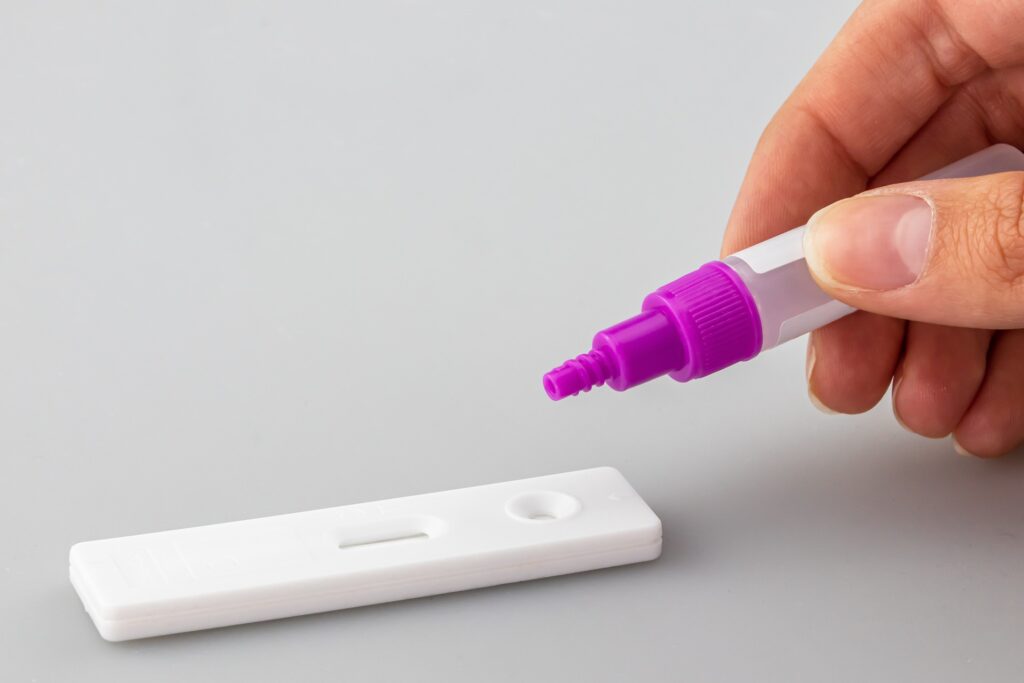
Last Friday, the U.S. Food and Drug Administration issued an emergency use authorization for the first over-the-counter (OTC) home diagnostic test that can differentiate and detect influenza A and B, as

Last Friday, the U.S. Food and Drug Administration issued an emergency use authorization for the first over-the-counter (OTC) home diagnostic test that can differentiate and detect influenza A and B, as